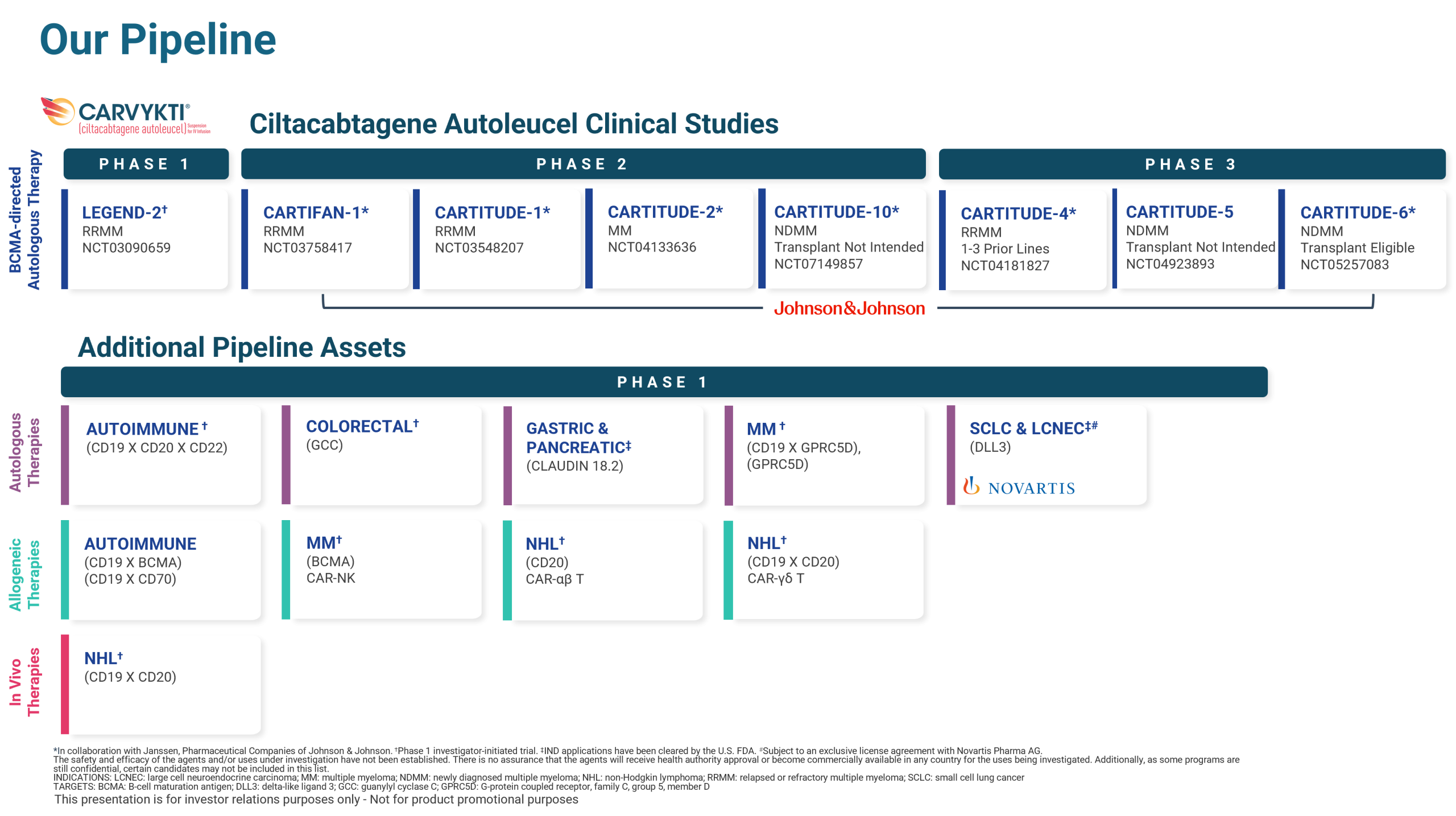
Pipeline
Our pipeline is made up of investigative therapies and innovative technologies in personalized medicine. We are devoted to exploring the potential of cell therapies to treat diseases that are considered intractable and incurable, such as hematological malignancies and solid tumors.

You are about to leave LegendBiotech.com
You are going to a website that Legend Biotech does not operate and to which Legend Biotech's privacy policy does not apply. Legend Biotech is not responsible for the content, format, maintenance or policies of the website you are about to visit and does not endorse or monitor any content on such website.



